The title, as I’m sure you have already recognised, is from a well-known strand in schooldays humour. This particular example came from a medical student, and I’ll keep the punch line until later.
From the submicroscopic level to international social policy, medicine has become a scientific computing dominated domain. Electronic data handling approaches are centrally responsible for increases in reach, effectiveness and efficiency, though also for dramatic growth in health programme delivery costs.
The highest persistent profile belongs to genetic areas, which could not exist without a mature substrate of computing technologies upon which to build. This doesn’t just mean computerised study leading to fundamental knowledge about genetics, but more traditionally pragmatic correlational studies such as the linkage between leptin expression and DNA methylation or MMP-13 activity in chondrocytes[1] (pointing to potential osteoarthritis therapies), or between gene variants at a particular chromosome locus and incidence of asthma[2]. Then there is the ascendancy of in silico methods for biological chemistry at the molecular level; computing is now so much a part of biology as to be inseparable from it and medicine, a consultant at a large hospital told me briskly over a cellomics display, ‘is only applied biology’.
Close on the heels of genetics comes epidemiology. Thrust into public controversy by press stories of the ‘where is our global bird flu pandemic, and when can we confidently expect one?’ variety, mathematical epidemiologists find themselves portrayed as ‘detectives ... soldiers and heroes ... liars’ and their computer models as tools to map or distort the truth[3]. From polio resurgence to MRSA and other bacterial acquisition in hospital settings, they are kept busy.
AIDS and HIV virus are primary targets for epidemiological modelling, and for other data analytic operations as well. Investigation of links between differential patterns of HIV infection and biological or demographic indicators, whether within subcommunities or across continents, is both medically vital and data intensive. Accurate estimation of incubation period is vital to the determination of intervention timetables, with treatments which can be aggressive and need to be minimised as far as possible. As with all modelling, constant attention to assumptions is crucial if costly (in resource and human terms) errors are to be avoided; the wide use of a Weibull model for virus incubation periods, infection distributions, survival, and so on, is a case in point. Running differential equation solutions in Wolfram Research’s Mathematica, Robert Byers and others at the Centers for Disease Control and Prevention have been able to show that Weibull predicts significant characteristics of observed data (including a seven-year plateau effect) less well than logistic alternatives.
For most consumers of medical services, diagnosis and treatment is of far more pressing concern than research, and here the biggest area of scientific computing application is in body scanning of various kinds. Regardless of their genesis (x-ray, ultrasound, magnetic resonance, radiometric tracking...), images are not just artefacts in their own right, but source material for mathematically constructed and computationally intensive three-dimensional informational models – effectively illustrated by Lund university’s IDL powered dosimetry study in last year’s March issue[4]. The faster the data processing hardware and software behind the scenes, and the more there is of it, the more detailed and responsive can be the reconstructed visualisations used by the physician.
Then there are broader strategic aspects of medical care, the application of industrial business methods to healthcare problems. Synthesis of research results into therapeutic outcomes, by modelling demand, shapes resource provision; quality control assures safety and predictability of that provision in deployment.
Quality control and assurance have, of course, to be strict and ubiquitous in a domain where errors can be fatal. Drugs that save or take lives depending on purity and close toleranced dosage are an obvious example, but even a humble saline solution needs to be kept closely within control limits if large-scale health operations that use them as a fundamental given are to function smoothly and transparently.

Opthalmic surgery under microsurgical manipulators developed at Columbia University’s Advanced Robotics and Mechanism Applications (ARMA) laboratory. (Image supplied, and copyright, by ARMA).
Almost all components of a properly functioning developed world medical system apply QA regimes that will be instantly recognisable to anyone familiar with modern manufacturing.
Blood banks, crucial to many aspects of medical intervention from operating theatre to field emergency, as essential and as potentially lethal as anaesthetics, are a representative example. The Welsh Blood Service is typical, using NWA Quality Analyst as the software basis for its assurance operations with ISO 9001 among the clutch of accreditations and protocols essential to its smooth running. Simple quality assurance isn’t the end of it, though; Quality Analyst also supports efficiency measures and thus the maximising of operational effectiveness. Programming of previously manual tasks to automate data entry and provide detailed documentation reduces opportunities for human error. Monitoring of markers at all process levels and feeding back the results as action triggers ensures that most problems are dealt with before they become significant, avoiding costs and errors in the workflow itself. Similar attention to degradation data promises both tighter standards and extended equipment life.
Adoption by Harvard’s Dana-Farber Cancer Institute of a tools suite from business intelligence specialists Inforsense underlines the point – as does the position of healthcare as second item in a list of five strategic areas (the others are, in order, pharma/biotech, finance, manufacturing and communications) for Inforsense itself. Their Translational Research Solution package handles all the problems of marrying disparate data sources, inconsistent formats, and origin types, to provide a single information platform upon which both research and intervention can be based. As with the blood bank, improved process efficiency is not just an end in itself but translates directly into effectiveness and patient benefit.
A particularly down-to-earth application (despite its science fiction associations) of scientific computing approaches and technologies, and one with a range and spread that seem certain to expand over the foreseeable future, is telemedicine.
Telemedicine is not, in essence, a new idea. Thirty years ago, as a medically-ignorant statistician working in sparsely populated areas of the Middle East and Africa, I was regularly asked to act as the eyes and ears of a medical professional on the other end of a radio link. Australia’s Royal Flying Doctor Service ‘medicine, aviation and radio were combined to bring health care to ... remote areas’ sees its 80th anniversary this year. Only with the arrival of computerisation, however, both as communications medium and as medical science resource, has it moved to centre stage as a strategic concept. There are two basic views of telemedicine: extending the reach of medicine into areas geographically remote from medical expertise, on the one hand, and increasing the cost-effectiveness of high capital methods on the other. In principle, both models come down to maximising the operational envelope of concentrated centres; in practice, they differ considerably and the second view is of more interest from a scientific computing standpoint.
Once an image (an x-ray, for example, or a conventional photograph of a lesion) is in digital form, it becomes more than itself; it is part of a virtual medical environment independent of location. This, in turn, means that processing power in one place can be applied to research, diagnosis or treatment at another. A monk in an African monastery, with no formal medical training and only a small donated dental x-ray machine to work with, describes how multiple small images of puzzling spinal injuries to several newborn babies were compiled under video link guidance from doctors in Texas. The x-ray images were scanned, digitised, and synthesised by a computer in Scotland to produce a single phenomenological model. Obstetricians in Sweden then gave advice that, translated into prenatal education, led to reduced infant mortality and malformation.
Texas, the Scottish highlands and islands, rural Africa and the Swedish wildernesses, are all areas where telemedicine can bring great effectiveness returns on investment. Texas, a predominantly rural state within the world’s most technologically advanced country, where almost one county in four has no hospital and nine per cent have no doctor, illustrates the value of distributed expertise. A developing programme of telemedical links makes advanced facilities (including analytic capacity and computer-based knowledge systems) available to the local GP, the prison infirmary, the peripatetic midwife and therefore, ultimately, to the population. Much of Africa shows the same pattern in greater degree, but with added factors ranging from under capitalisation through wider geographical distribution and thinner technological infrastructures to fear of aid with strings attached.
The implementation and impacts of telemedicine, in either the developed or developing worlds, is patchy as yet; Richard Wooton, of the University of Queensland’s Centre for Online Health, estimates[5] that ‘only about 0.1 per cent of the potential telemedicine demand from the developing world is being met’. Nevertheless, so-called ‘e-health’ provision programmes are already on offer in fields as diverse as cytology[6], HIV/AIDS antiretroviral management[7], cardiology, malaria diagnosis and ophthalmology[8]. Planning for future medical contributions by the developed world into the developing is firmly predicated on this route. E-health provision is one of the factors credited with powering transformation of developing world societies, such as Pakistan[9], from peasant agrarianism to information bases.
Throughout industrialised societies, these resource sharing possibilities are increasingly leading to change in the organisation and perceived face of medical practice. The ‘polyclinic model’ is well established in the US and some European countries, and the clear basis of healthcare development planning in Britain and elsewhere.
A well developed case of internal telemedicine, though not usually referred to as such, is the already mentioned move to digital x-ray images within health services. Dr Brian Corden, a paediatrician in Maryland with a strong research background who implemented one of the early computerised hospital record systems, comments that ‘digital x-rays now fly around the country on a daily basis. If I have a tough case I get up our local x-ray on my computer screen, copy the pertinent shots, and send them off to an orthopaedic or urology specialist to take a gander.’ With images in this form, other technologies become viable – starting with pattern recognition. Electrocardiograms are already routinely machine interpreted; other areas are certain to follow. ‘Radiology,’ comments Dr Corden, ‘is a field in jeopardy. I see no reason why we don’t have software that recognises a pneumonia on x-ray.’

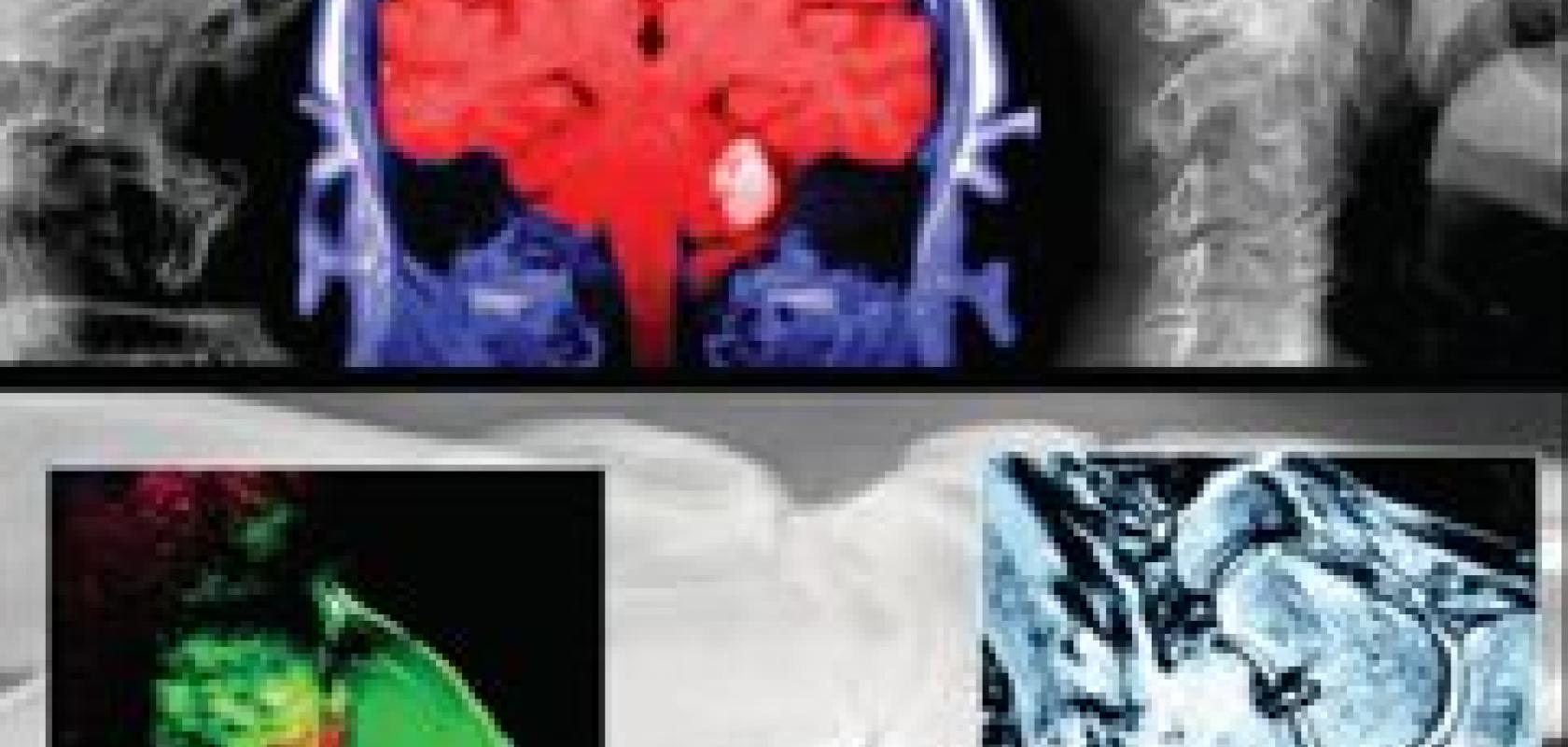
A computed tomography (CT) scan of a patient with lung cancer and pleural effusion, showing (A) lung metastases, (B) the heart, (C) collapsed lung and (D) effusion. (Image supplied by Brian Corden, MD).
Prosthetics are an area where data analysis is increasingly intensive, though since this overlaps with haptic robotics in the next issue I’ll not go overboard on it. Devices such as an embedded telescope for patients suffering end-phase, age-related macular degeneration[10], prototype artificial limbs controlled directly from the motor cortex[11], or studies aimed at self-monitoring powered servo mechanisms are all dependent on implementation of high level analytic methods. Robotics are rapidly making inroads elsewhere, as well, with Da Vinci machines well established in minimally invasive cardiac surgery and spreading to other areas, such as Dr Corden’s example of paediatric urology[12]. Beyond (or in combination with) the bulky external Da Vinci machines there is a vigorous line of development towards multiple miniature robots that will operate entirely in vivo (for example, within the peritoneal cavity[13]). Each robot is, so far, expected to be specialised in providing a single function (lighting, vision, manipulation, and so on) and the future seems most likely to lie with this sort of cooperative ‘swarm’ approach, although I have been shown some generalist prototypes for use in larger environments, such as the colon.
The most basic level of computerisation and data analysis in medical practice is one which, for that reason, I haven’t yet mentioned: transition from paper to electronic patient records. In efficiency and clientbenefit terms it is perhaps also one of the most potentially productive changes as well – information is the doctor’s most valuable resource at the sharp end, and anything that increases its efficient and appropriate delivery can only be a good thing. Implementation, however, has been anything but smooth.
In the US, three decades or more after the first systems were put in place, the market is still fragmented and records on one vendor’s system are incompatible with another – and if a system goes down, the records are not even accessible using generic methods or tools. To make things worse, hospital systems are different from individual practitioner setups. In the UK, where the model is different and standardisation easier in principle to specify, problems come from the opposite direction: the size of the resulting system that has to be realised by multiple suppliers. Fujitsu has just pulled out of its involvement in the scheme amid a dispute over implementation in a programme which is already four years behind schedule.
Moving up the technology ladder, inserting a new method or device (and its associated new skill demands) into an existing medical system creates a gravitational distortion in the surrounding financial structures. Dr Corden comments that ‘In our small community there are at least four MRI scanners. There is tremendous competition for patients and tremendous resistance by insurance companies to pay for scans ... [technology] it is one of the largest contributors to the cost of medicine in the US which is so out of control that we will probably crash before it is fixed.’
A generation or two from now, history suggests, the problems will have ironed themselves out and only the benefits remain as cutting edge (no pun intended) becomes routine. In the mean time, that joke from my medical student goes like this... A man comes into the surgery, and says ‘Doctor, doctor – I think I’m a computer!’ To which the doctor replies, ‘Sit down here, then; mine crashed a week ago, and I’ve a backlog of work to process.’
References
1. Iliopoulos, D., K.N. Malizos, and A. Tsezou, Epigenetic regulation of leptin affects MMP-13 expression in osteoarthritic chondrocytes: possible molecular target for osteoarthritis therapeutic intervention. Ann Rheum Dis, 2007. 66(12): p. 1616-1621
2. Konstantinidis, A.K., et al., Genetic association studies of interleukin-13 receptor {alpha}1 subunit gene polymorphisms in asthma and atopy. Eur. Respir. J., 2007. 30(1): p. 40-47
3. Nerlich, B., Glossary of terms: Media, Metaphors and Modelling: How the UK Newspapers Reported the Epidemiological Modelling Controversy during the 2001 Foot and Mouth Outbreak. Journal of Experimental Biology, 2007. 210(9): p. 1492-1496
4. Grant, F., If a picture paints a thousand words, in Scientific Computing World. 2007
5. Wootton, R., Telemedicine support for the developing world. J Telemed Telecare, 2008. 14(3): p. 109-114
6. Malami, S.A., Use of telemedicine for cytology training in Africa. J Telemed Telecare, 2008. 14(2): p. 105-106
7. Sørensen, T., U. Rivett, and J. Fortuin, A review of ICT systems for HIV/AIDS and anti-retroviral treatment management in South Africa. J Telemed Telecare, 2008. 14(1): p. 37-41
8. Baker, H., C. Ntim-Amponsah, and I.E. Murdoch, A survey of patients attending an eye clinic in Ghana. J Telemed Telecare, 2007. 13(8): p. 397-400
9. Shafique, F. and K. Mahmood, Indicators of the Emerging Information Society in Pakistan. Information Development, 2008. 24(1): p. 66-78
10. Colby, K.A., et al., Surgical Placement of an Optical Prosthetic Device for End-Stage Macular Degeneration: The Implantable Miniature Telescope. Arch Ophthalmol, 2007. 125(8): p. 1118-1121
11. Velliste, M., et al., Cortical control of a prosthetic arm for self-feeding. Nature, 2008
12. Volfson, I.A., et al., Robot-assisted urologic surgery: safety and feasibility in the pediatric population. J Endourol, 2007. 21(11): p. 1315-8
13. Lehman, A.C., et al., Surgery with cooperative robots. Comput Aided Surg, 2008. 13(2): p. 95-105
Thermo Fisher Scientific - Cellomics, info@cellomics.com
Lund University - LundADose project, info@info.lu.se (general), kansliM@kansliM.lu.se (faculty of medicine)
Wolfram Research - Mathematica, Finance Essentials package, Mathematica link for Excel, info@wolfram.co.uk
Adept Science - NWA Quality Analyst, info@adeptscience.com
Biosoft - Quantiscan, info@biosoft.com
Inforsense - Translational research solution, http://www.inforsense.com/company/contact/
IDBS - XLfit, info@idbs.com