The healthcare industry has been slow to adopt engineering simulation compared to other sectors.
However, an intrinsic need for modelling and simulation has become increasingly apparent as medical technologies continue to advance. Valerio Marra, marketing director at Comsol, explained: ‘In an industry where safety is of paramount importance, the capability to investigate different scenarios by specifying boundary conditions, material properties and physiological mechanisms allows for early and harmless correction of design mistakes.’
The medical devices sector is now taking steps to integrate modelling and simulation into its design and development processes. Paul Goossens, vice president of engineering solutions at Maplesoft, said: ‘While system-level modelling is something that companies in other industries, such as aerospace and automotive, have been using for decades, it is becoming a more popular tool in the medical device market.’
Goossens added: ‘One of the driving factors is the current increase in safety and functionality issues within the medical device industry, and the growing concern surrounding product recalls. Products are removed from the market for several reasons and the complexity of many of these devices requires an early diagnosis in the design process, which is where early insights and diagnosis in system behaviour can allow for safer products without the time and costs associated with the standard cycles of medical device testing.’
Simulation and modelling reduce the lengthy timescales and high costs often associated with medical device developments. Medtronic, which makes such instruments, recently claimed the use of computer models helped reduce its time to market by two years and cut the cost of clinical trials by $10 million for a specific treatment.
Many practical issues associated with real medical tests can be overcome. Marra added: ‘Especially for medical devices, the fact that simulation results can be accessed in locations where it would be impractical (if not impossible) to place sensors on a physical prototype, or in the human body, is greatly appreciated.’
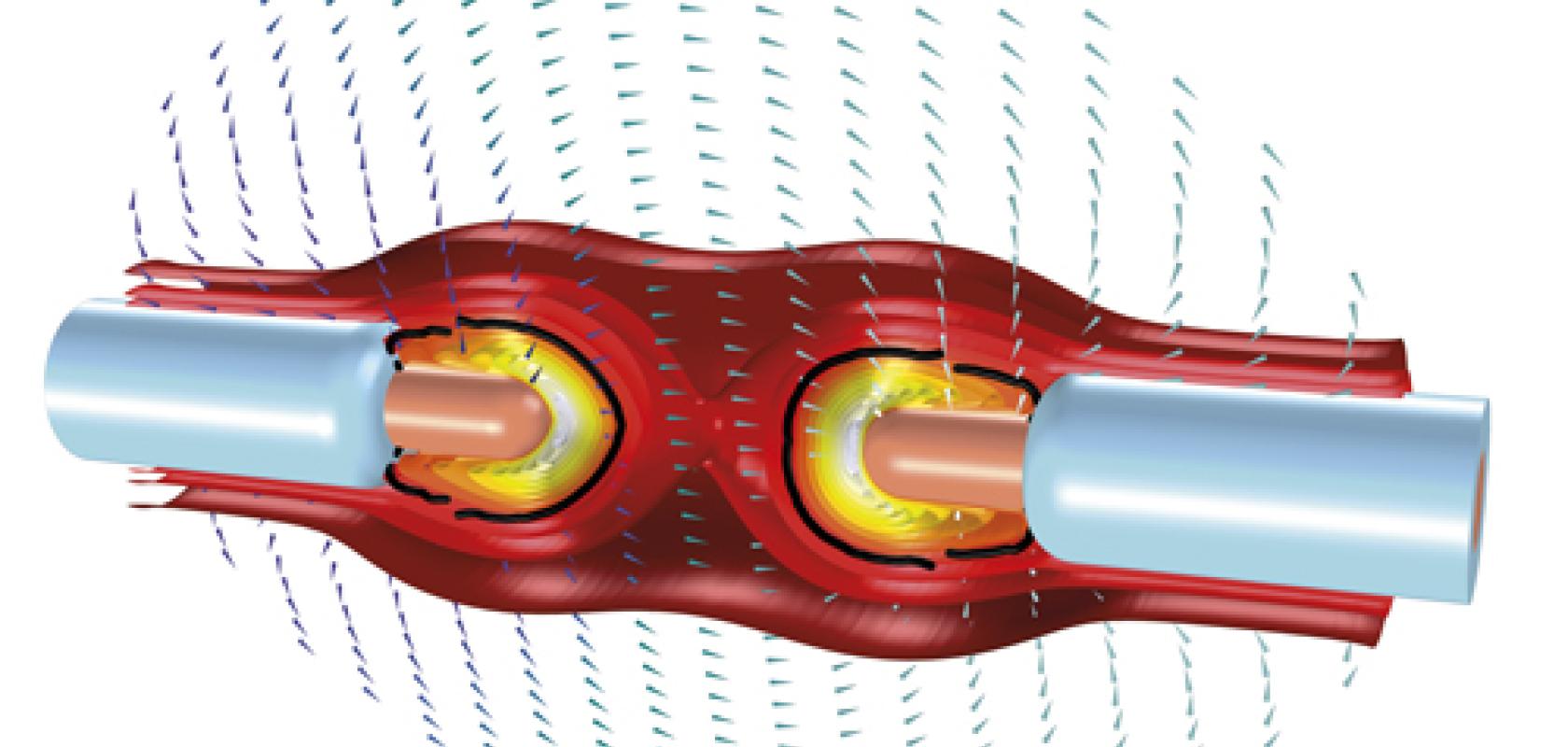
Multiphysics simulation can boost medical device design in many ways, according to Marra. It reduces the need for physical prototyping, makes available the measurements of any modelled variable at any point in a medical device and its surroundings, and provides high-fidelity medical device modelling.
However, a range of challenges still needs to be addressed and managing the complexities of both the human body and the medical devices is paramount.
Regarding the complexity of the medical devices, Goossens explained: ‘A typical approach for the development of multi-domain systems often carries the risk of high costs and time-consuming re-engineering, due to the lack of interoperability between different domains.
‘From powering systems to the mechanical, electrical and fluid components involved in medical devices, these multi-domain systems present many challenges, not only because of the complexity in modelling the related sub-systems, but also when it comes to interfacing each of these sub-models into one single integrated model,’ Goossens added.
When it comes to the complexities of the human body, Thierry Marchal, global industry director for sports and healthcare at Ansys, said: ‘This never-ending challenge requires more research and investment to improve the models in their accuracy, long-term predictability and ensure we fully understand the limitations of the current models.’
The human body is also a very different environment compared to the systems regularly simulated in established industries, such as aerospace or automotive. Milad Mafi, product marketing manager at SimScale, said: ‘The biggest challenge from a simulation setup perspective is definitely geometry modelling. Simulation has its roots in classical mechanical engineering and all concepts and processes developed in recent years are designed for technical geometries and materials.’
A good example is the material characterisation of steel compared to bone. Mafi explained: ‘While metallic materials can be described by isotropic material models, this is often not possible with biological materials. The bone gains high stiffness from the complex microstructures, which move in geometric scales of a few micrometres and cannot be resolved with current methods. It is, therefore, necessary to supply replacement models that are both technically and medically suitable.’
Restrictive regulations
These models also need to be validated to demonstrate that the model does accurately predict what will happen in the human body for various patients. Ansys recently collaborated with medical device companies, under the guidance of the FDA and ASME regulatory bodies, to address this point and develop new standards for the verification and validation of medical devices.
Such close collaboration between simulation companies and regulatory authorities could help accelerate adoption in the medical devices market. Ansys has also collaborated with numerous hospitals around the world and regulatory authorities in Europe, the USA and Asia. Marchal said: ‘This [collaboration with regulators] is helping us understand what these local agencies and European Notified Bodies require in terms of model validation and results format to accept simulation results as evidence for their respective regulatory approval.’
It’s not just collaboration with regulators that will help drive further adoption of simulation and modelling in the medical devices arena. Simulation experts and device designers are also collaborating at a growing rate.
Such cross-discipline work is facilitated through simulation applications, according to Marra, who added: ‘Such apps allow the users to input the parameters needed to get the information they need, the stress in a bone structure or the temperature rise during an ablation process, without having to deal with the complexity of a multiphysics model, something their fellow simulation specialists took care of for them.’
SimScale has also developed a production-ready SaaS application for engineering simulation. It provides instant access to computational fluid dynamics and finite element analysis via a user-friendly web application. Mafi explained: ‘In general, our users use simulation with SimScale to test cardiovascular stents, hip joint or arm prostheses, disposable pumps, in vivo blood flow or laboratory equipment.’
Abundant applications
A wealth of application areas are now emerging in the medical devices sector. Mafi added: ‘In my opinion, the leading star is the availability of patient-specific implants. Especially in the treatment of diseases of the cardiovascular system, such as arteriosclerosis but also in the treatment with joint replacement, simulation offers an unbelievable potential. FEA and CFD analysis play a decisive role in this context and will increasingly act as a catalyst in the coming years.’
Simulation and modelling are also helping the healthcare industry develop artificial biocompatible organs, such as an artificial heart, kidney and pancreas. Marchal explained: ‘These devices are expected to be implanted into patients for an extended time. Thanks to simulation, their size, weight and necessary energy to support them are now compatible with patients’ and medical professionals’ expectation. This solution may soon solve the problem of lacking organ donors.’
Another emerging area is the adoption of simulation in surgical planning. Marchal explained: ‘Creating a computer model of part of a patient gives the surgeon the luxury to test different surgical approaches, identify potential problems and select the best method before entering the operating room.
‘This new approach is greatly relieving the stress from the surgeons, who can now quietly investigate various solutions without the stress of the patient waiting on the table.
‘This also improves the outcome of the surgery, helping reduce the recovery period and sometimes helping avoid dramatic situations,’ Marchal added.
Rise of the robots
The use of robotics in medical devices is another evolving area, according to Goossens. In the biomechanics field, a team of researchers led by Dr Andrew James Smith, at York University, have used MapleSim to model autonomous battery operations in humanoid robots and electrical assistive devices.
Goossens said: ‘The research group undertook the task of determining at what point in the transitions between sitting and standing energy can be regenerated in an orthosis or prosthesis, much like how a hybrid vehicle regenerates energy during braking by drawing it from the motor for re-use in the vehicle’s operation.’
Biomechanical data from human trials was used to provide the desired trajectories for the simulations in a multi-domain model in MapleSim, allowing the robotic model to mimic human movements when transitioning between sitting and standing positions. Goossens said: ‘The group’s findings have a meaningful application for prostheses and orthoses design, and determining the most efficient battery autonomy means the operation time of these devices can be extended, and smaller, lighter batteries can be used, reducing their bulk. Ultimately, a more efficient device can reduce joint loads during standing-to-sitting for users – critical for people suffering from joint diseases.’
Robotic-assisted surgery is another developing field, which allows precision robotic tools to act like a surgeon’s arms, hands and fingers, allowing surgeons to reach areas the human hand cannot reach without making large incisions.
Surgeons using haptic technology can control the movement of a robotic arm, but when they strike an obstacle during a surgical procedure, they feel the force of the obstacle against their hand and can take steps to avoid it.
Engineers at Quanser used Maple to develop the controllers both for the robot motion and for the haptic feedback to a surgeon’s hands. The engineers first modelled the behaviour of the mechanism and, using Maple, developed the systems of differential equations that modelled the kinematics and dynamics of the system.
Goossens said: ‘Once this was done, the team could very quickly test their model by solving the equations of motion, and develop the control strategies within the Maple environment. The work in this area has far-reaching and exciting implications for the future of robotically-assisted surgery, such as brain microsurgery, nanosurgery and telesurgery. The time for remote surgery with a sensory element is definitely here.’
Digital twins
Digital twins are a popular emerging application area in the medical devices arena. Here, a computer model of the patient can test new devices before the real patient receives the device.
Goossens explained: ‘[A digital twin] creates the perfect companion for diagnostics, maintenance and product innovations. Modern design tools can create model-driven digital twins to assist in all stages of product design, and because it doesn’t need test data to predict behaviour, it can be used for conceptual design before any physical prototype of a device has been constructed.’
This has positive implications for the patient, as Marchal explained: ‘This allows medical professionals to implant medical devices and assess the impact on your body, or they can give your digital twin some medicines and assess how your body would react to specific doses.
‘This is opening the door to predictive medicine which will be able to predict what could happen to you, and also preventative medicine able to suggest the treatment to prevent health issues before they impact your life. When digital twins become a widespread reality, we expect that the cost of healthcare will dramatically decrease, while each of us will enjoy a longer and healthier life,’ Marchal added.