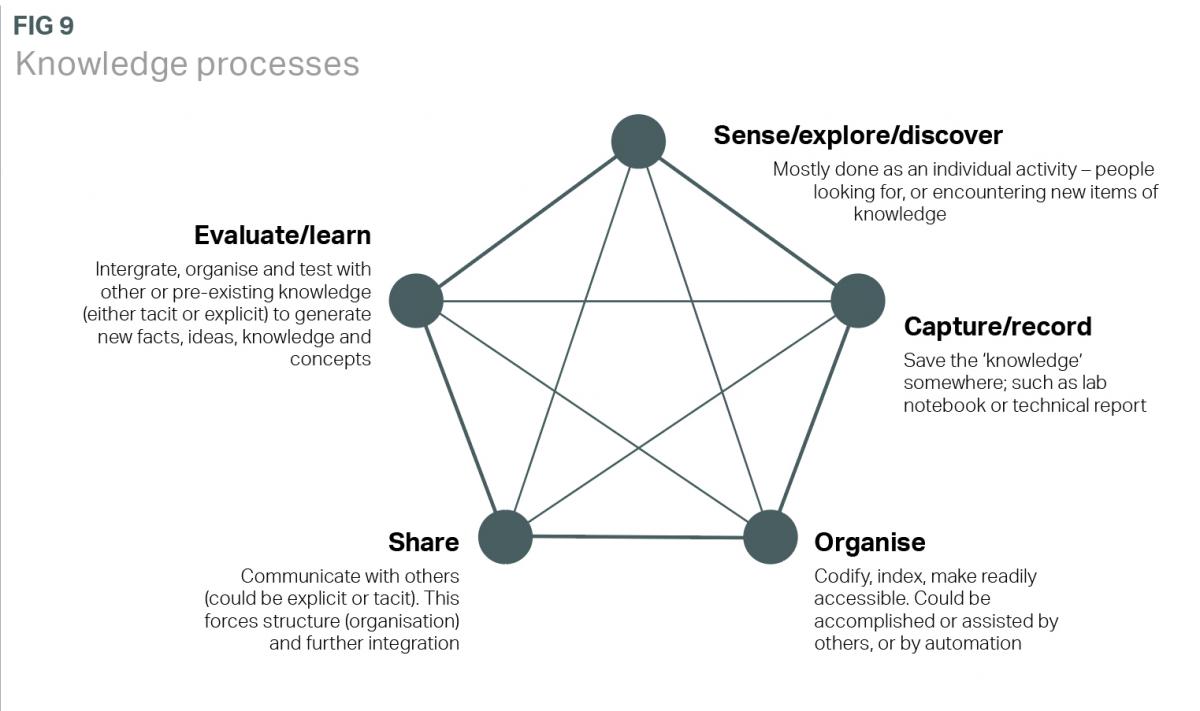
The five nodes in Figure 9 represent a generalisation of major knowledge processes. It is evident that technology, in laboratory informatics tools, has an enabling role in a knowledge ecosystem.
From laboratory informatics to knowledge management, technology is predicated on logical and systematic processes. But serendipity has always had a significant role in science. Many advances have originated from ‘what if’ moments, chance observations, and things that went wrong. Failure often has more to teach us! With a growing emphasis on right-first-time, error-reduction and productivity, it is a management challenge to take time to review successes and failures.
The role of the informatics tools in a smart laboratory, or ‘knowledge ecosystem’ (Figure 9) is important. They are strong in terms of capturing, recording and organising data, and increasingly, they provide facilities for sharing information. But further opportunities arise with regard to evaluation and learning (data analytics) and experimental design. Further afield, they will contribute to predictive science.
Nevertheless, there needs to be some space for ‘right brain’ thinking, alongside those systematic and structured approaches for increasing efficiency and productivity. Innovation depends on knowledge and understanding. Although technology can assemble and look after the data, making sense of it is down to human assessment and understanding. In the main, so-called knowledge management ‘solutions’ are no more than data or information management solutions.
It’s only when the human component is added that knowledge management can flourish, and even then, it needs the right environment – hence, the concept of a ‘laboratory ecosystem’, or smart laboratory. Although management may want to see such an ecosystem, it can buy only the tools. It must create the right environment for the ecosystem to be nurtured and cultivated.
The ecosystem is dependent on an open and collaborative culture and supportive leadership; not secrecy, discipline or rigid management. Participants need to opt in; not be forced in. One worry is that the digital revolution may be driving a lot of thinking to be ‘digital’, with the risk that random, analogue mindsets and gut feelings may be seen as irrelevant and inconsistent with modern concepts of science.
This way of looking at things may shed some light on why the early ELN market was sub-divided into different solutions for chemistry, biology and QA.
The risk for a multi-disciplinary laboratory that is looking to implement an ELN would be to adopt a one-size-fits-all approach. This could generate disaffection among users.
The current informatics market is moving to more modular solutions, with a generic core and optional discipline-specific modules. This creates a better opportunity to find a single-source solution. The shared functions can be separated from scientific functions, which are closer to the soul of the scientist’s laboratory work. Shared functions would include issues like document authoring, approval/witnessing, file and document management, and legal and regulatory compliance. All fall into the ‘bureaucratic’ category and lend themselves to process improvement opportunities, more readily than the scientific aspects. It may still be a one-size-fits-all approach, but it can be designed to accommodate the requirements of multi-disciplinary laboratories, and to standardise and improve common sub-processes, rather than making compromises.
Ideally, laboratory informatics tools should not be perceived as an intrusive bureaucratic process, but rather as something that facilitates the scientific method and doesn’t intrude on the social and intellectual processes that are essential to the science. Achieving this objective is essential to joined-up science and to user acceptance, and is a responsibility that falls to management in its objective of building a smart laboratory. It requires a sympathetic view of the requirements of the different disciplines, and the way in which these functions are managed and provided for, even when organisational demands push for increased uniformity and consistency.
The concept of a smart lab varies from organisation to organisation depending on the business, and its technological choices. Discovery and development are increasingly recognised as steps in a holistic product lifecycle process, rather than separate functions.
The focus of this guide has been on technology, with due consideration to the laboratory processes to which it can be applied. It has also touched on aspects of culture and technology adoption, but it must be remembered user acceptance is critical in almost every system or project.
Technology on its own cannot overcome challenges in the laboratory. To become ‘smart’, lab users and managers need to understand their role in an organisation’s end-to-end business processes, and optimise its technologies to fulfil those requirements.
References:
The Gartner Hype Cycles: www.gartner.com
CENSA: The Collaborative Electronic Notebook Systems Association
Good Manufacturing Practice (GMP): The US has one set in theFederal Register 21 CFR, and the EU has its own, as do other geographical areas and organisations like the Organisation for Economic Co-operation and Development (OECD) US FDA, 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals, 2005, FDA: www.fda.gov European Union Volume 4: Good Manufacturing Practices – Medicinal products for human and veterinary use, 1998, 153 pages, incl. Annex 11 covering computerised systems
Good Laboratory Practice (GLP): The US has one set in the Federal Register 21 CFR; EU has its own, and also other geographical areas US FDA, 21 CFR Part 58 Good Laboratory Practice for Non-Clinical Laboratory Studies, 2005, FDA: www.fda.gov European Union, Council Directive of 7 June 1988 on the inspection and verification of Good Laboratory Practice (GLP) (88/320/EEC) European Union, Council Directive – of 24 November 1986 - 86/609/EEC – on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes
US FDA, 21 CFR Part 11 Electronic records; electronic signatures, 1997, FDA: www.fda.gov OECD Series on principles of good laboratory practice and compliance monitoring number 10
European Union Volume 4: Good Manufacturing Practices – Medicinal products for human and veterinary use, 1998, 153 pages, incl. Annex 11 covering computerised systems
PIC/S, PI 011-03 Good practices for computerised systems in regulated ‘GxP’ environments. 25 September 2007, PIC/S: www.picsscheme.org
GAMP 5 (Good Automated Manufacturing Practice) Guide: A Risk-Based Approach to Compliant GxP Computerized Systems, February 2008, International Society for Pharmaceutical Engineering (ISPE), Fifth Edition, ISBN 1-931879-61-3: www.ispe.org
Good Automated Manufacturing Practice Guidelines version 5, International Society for Pharmaceutical Engineering, Tampa FL, 2008 McDowall, R.D., (2009) Spectroscopy Focus on Quality, p23
International Society for Pharmaceutical Engineering GAMP 5: Ten Years On https://ispe.org/pharmaceutical-engineering/may-june-2018/gamp-5-ten-ye…
Using Electronic Records in Patent Proceedings, article by Damien McCotter and Peter Wilcox. Originally published in Managing Intellectual Property’s World IP Contacts Handbook, 14th edition, 2007. Available at www.mondaq.com
IP Expert Advice: Tips on creating a lab notebook that contains ‘convincing evidence’: www.edn.com/article/CA6445886.html?industryid=47048
Admissibility of Electronic Records in Interferences, Bruce H. Stoner Jr., Chief Administrative Patent Judge, www.uspto.gov/web/offices/com/sol/og/con/files/cons119.htm
Private communication: Colin Sandercock (Perkins Coie LLP) September 2011
Pistoia Alliance AI & Deep Learning Papers, Links, Articles - https://pistoiaalliance.atlassian.net/wiki/spaces/PUB/pages/108396550/A…
The ABCs of Electronic Signatures, Nettleton, D., Lab Manager Magazine, 9 September 2010: www.labmanager.com/?articles.view/articleNo/3800/title/The-ABCs-of-Elec…
Rogers, E. M., Diffusion of Innovations, The Free Press. New York
Moore, G. A., Crossing The Chasm, Capstone Publishing
Bagozzi, R. P., Davis, F. D., and Warshaw, P. R., (1992). Development and test of a theory of technological learning and usage. Human Relations, 45(7), 660-686
Multi-Ontology Sense Making, David Snowden, http://cognitive-edge.com/uploads/articles/40_Multi-ontology_sense_maki…
Further reading and websites
Stafford, J. E. H., (1995) Advanced LIMS Technology: Case studies and business opportunities, Springer
Christensen, C. M., (1997) The Innovator’s Dilemma: When New Technologies Cause Great Firms to Fail, Harvard Business School
Segalstad, S. H., (2008) International IT Regulations and Compliance: Quality Standards in the Pharmaceutical and Regulated Industries, Wiley-Blackwell
McDowall, R. D., (1987) Laboratory Information Management Systems, Sigma Press
Laboratory Notebook Guidelines: BookFactory, LLC, 2302
S. Edwin C. Moses Blvd, Dayton, OH 45408. Available at
Mahaffey, R. R., (1990) LIMS: Applied Information Technology for the Laboratory, Nakagaw
Sellen, A. J., and Harper, R. H. R., (2003) The Myth of the Paperless Office, The MIT Press
Franklin, C., (2003) Why Innovation Fails, Spiro Press
Kanare, H. M., (1985) Writing the Laboratory Notebook, An American Chemical Society Publication
eOrganizedWorld: www.eorganizedworld.com
Free online LIMS training courses: www.LIMSuniversity.com
The Generally Accepted Recordkeeping Principles:
Independent, non-commercial LIMS user’s group:
Industrial Lab Automation: www.industriallabautomation.com
The Institute for Laboratory Automation: www.institutelabauto.org
The Integrated Lab: www.theintegratedlab.com
Journal of Information & Knowledge Management (JIKM):
www.worldscientific.com/worldscinet/jikm
LIMSfinder: www.LIMSfinder.com
NL42 Consulting: www.NL42.com
Online encyclopaedia for laboratory, scientific and health informatics: www.LIMSwiki.org
PDF/A standard: http://en.wikipedia.org/wiki/PDF/A
Scientific Computing World: www.scientific-computing.com
Segalstad Consulting: www.limsconsultant.com


